Laparoscopic Warshaw procedure in pediatric solid pseudopapillary neoplasm of the pancreas: Technical feasibility and oncological outcomes
Abstract
Achieving margin-negative resection is crucial in treatment of solid pseudopapillary neoplasm (SPN) of the pancreas, while preserving the spleen during distal pancreatectomy is highly desirable in pediatric cases. Laparoscopic Warshaw procedure (Lap-WT) is invaluable when tumor involvement in splenic vessels complicates preservation. However, the feasibility of Lap-WT in pediatric patients remains contentious. This study presents the clinical outcomes of three pediatric SPN cases who underwent Lap-WT. The median age was 10 years, with a median tumor size of 50 mm. Lap-WT demonstrated successful outcomes with a median operation time of 311 min and blood loss of 12 mL. No postoperative complications occurred, with a median length of hospital stay of 8 days. Long-term follow-up showed mild thrombocytopenia and increased spleen volume in two cases, perigastric varices in one, with no bleeding complications. No instances of tumor recurrence were observed. Lap-WT emerges as a feasible approach for pediatric SPN, ensuring spleen preservation without compromising oncological outcomes.
1 INTRODUCTION
Solid pseudopapillary neoplasm (SPN) of the pancreas is a rare, low-grade malignant neoplasm estimated to comprise 1%–2% of all exocrine pancreatic tumors. Although SPN constitutes the majority of pediatric pancreatic neoplasms, representing approximately 70% of cases among children, its overall incidence of SPN in this demographic is low, presenting distinctive challenges in their management. Although these low-grade malignant tumors typically require margin-negative surgical resection, achieving a commendable 5-year survival rate of over 95%, their occurrence in the left-sided pancreas of children requires a delicate balance between achieving curative resection and preserving the spleen due to its pivotal role in immune system.
Laparoscopic spleen-preserving distal pancreatectomy (DP) utilizing the Warshaw procedure (Lap-WT) has been reported to be a feasible technique for spleen preservation in adults with benign or low-grade malignant tumors. This technique is notably applicable when the tumor is enlarged or attached to the splenic vessels, thereby making the preservation of these vessels challenging.Conversely, reports specifically addressing the technical and oncological feasibility of Lap-WT for SPN among pediatric patients are extremely limited.
This study presents the clinical outcomes of three pediatric SPN cases who underwent Lap-WT, with a particular focus on assessing long-term clinical outcomes.
1.1 Case presentation
Three pediatric patients younger than 15 years diagnosed with SPN underwent Lap-WT at our institution. The indication for Lap-WT in these cases was determined by our institutional criteria, which consider factors such as low-grade malignancies and the presence of tumors exhibiting splenic vessel involvement as revealed by preoperative imaging. Table delineates the preoperative patient characteristics of the three pediatric cases diagnosed with SPN that underwent Lap-WT. The study included one male and two female pediatric patients with a median age of 10 years (range: 8–13). The median body mass index (BMI) was 17.5 (15.9–20.7) kg/m2, and the American Society of Anesthesiologists (ASA) physical status classification was class 1 for all cases. The median maximum tumor size was 50 mm (range: 28–66 mm). The tumors were located at the tail of pancreas, and the splenic vessels were compressed and deformed by the tumor in all cases (Figure ). Pancreatic transection was planned at the pancreatic neck in all cases. The median thickness of the pancreas at transection line, as measured on preoperative computed tomography (CT), was 7 (5–10) mm.
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Patient characteristics | |||
| Gender (male/female) | Female | Male | Female |
| Age (years) | 13 | 10 | 8 |
| Height (cm) | 148.7 | 135 | 137 |
| Body weight (kg) | 45.8 | 29.0 | 32.9 |
| Body mass index (kg/m2) | 20.7 | 15.9 | 17.5 |
| ASA physical status classification (I–VI) | Class I | Class I | Class I |
| Tumor location (body/tail) | tail | tail | tail |
| Tumor sizea (mm) | 66 | 50 | 28 |
| Transection line of pancreas (neck/body/tail) | neck | neck | neck |
| Thickness of pancreas at transection line (mm) | 5 | 7 | 10 |
| Perioperative outcomes | |||
| Operation time (min) | 344 | 311 | 244 |
| Estimated blood loss (mL) | 12 | 12 | 0 |
| Conversion to open surgery (yes/no) | No | No | No |
| Postoperative complication (C–D IIIa or more) (yes/no) | No | No | No |
| CR-POPF (grade B or C) (yes/no) | No | No | No |
| Splenic infarction after surgery (yes/no) | No | No | No |
| Postoperative infectious complications (yes/no) | No | No | No |
| Length of hospital stay (days) | 13 | 8 | 7 |
| Microscopic status of surgical margin (positive/negative) | Negative | Negative | Negative |
| Long-term clinical outcomes | |||
| Development of perigastric variesb (yes/no) | Yes | No | No |
| Gastric variceal bleeding (yes/no) | No | No | No |
| Complication of infectious disease (yes/no) | No | No | No |
| Occurrence of diabetes mellitus after surgery (yes/no) | No | No | No |
| Tumor recurrence after surgery (yes/no) | No | No | No |
| Observation period after surgery (months) | 79.6 | 44.0 | 29.9 |
Abbreviations: ASA, American Society of Anesthesiologists; C–D, Clavien–Dindo classification; CR-POPF, clinically relevant postoperative pancreatic fistula.
a Maximum diameter of the tumor.
b Tortuous veins lager than 5 mm in diameter along the outer edge of the gastric wall on the postoperative computed tomography image.
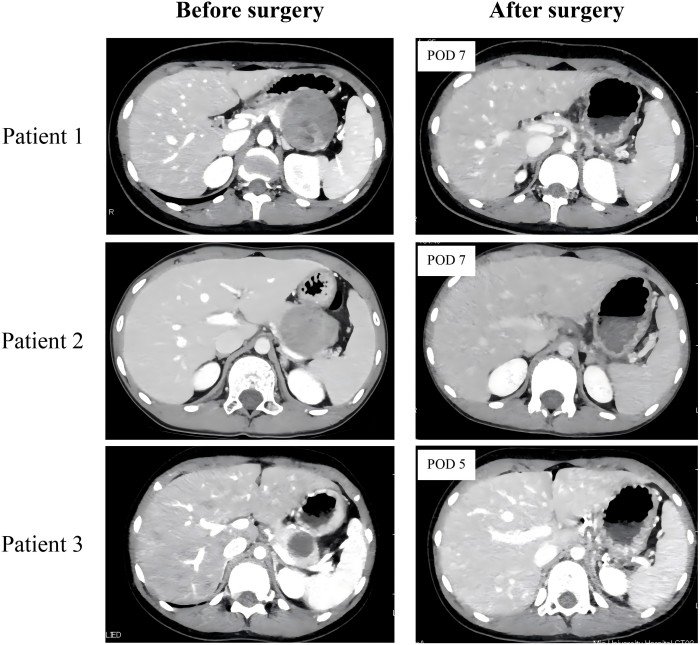
Preoperative and postoperative enhanced computed tomography (CT) findings in three patients undergoing laparoscopic spleen-preserving distal pancreatectomy with Warshaw procedure for solid pseudopapillary neoplasm. The enhanced CT scans verified adequate splenic perfusion, with no notable splenic infarction detected in any cases on postoperative day (POD) 5 or 7.
All surgeries were performed by a board-certified expert surgeon from the Japanese Society of Hepato-Biliary-Pancreatic Surgery and an endoscopic surgical skill qualification system qualified surgeon from the Japan Society for Endoscopic Surgery, employing standardized techniques (Y.M.). The patient was positioned in a supine reverse Trendelenburg position under general anesthesia. An open technique was employed to create pneumoperitoneum through a periumbilical 12-mm trocar under direct vision. Abdominal pressure was maintained at 10 mmHg by insufflating CO2 gas using the AirSeal system for all cases. Subsequently, two 12-mm, two 5-mm, and one 3-mm trocars were placed in the abdomen, as shown in Figure . The procedure initiated with the dissection of the pancreatic tail from the splenic hilum to isolate the distal splenic artery and vein, utilizing a tail-first approach (Figure and Video ). The distal splenic artery and vein were collectively encircled and taped proximally to the root of the left gastroepiploic artery and vein. For elevating the stomach, the antrum was retracted using a cloth tape, followed by lifting the gastropancreatic fold using 3 mm forceps. Additionally, the posterior wall of the upper body of the stomach was raised toward the abdominal wall using a straight needle. Subsequently, the root of splenic artery was isolated and divided. Pancreatic transection was conducted using a triple-row stapler with polyglycolic acid (Endo-GIA™ reinforced Reload with Tri-Staple™ Technology 60 mm combined with Signia™ Stapling System, Covidien, Tokyo, Japan). The black cartridge, allowing a postfire staple height of 4.0–5.0 mm, was used, and prefiring compression was routinely performed. Subsequently, the root of splenic vein was encircled and divided using a triple stapler (45-mm Endo-GIA™ camel load). Finally, the distal splenic vessels were divided using a triple-row stapler (45-mm Endo-GIA™ camel load) after lifting them up by a cloth tape. After removal of the resected specimen, a closed suction drain was placed in the peripancreatic and/or left subphrenic spaces.
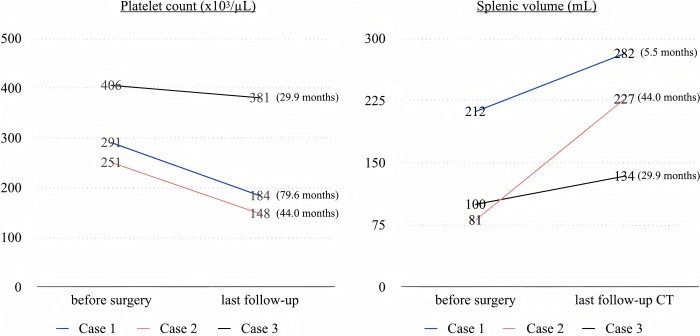
The transition of postoperative platelet count and splenic volume after surgery.
2 DISCUSSION
The clinical courses of the three pediatric SPN cases highlighted the technical and oncological feasibility of Lap-WT, emphasizing its ability for margin-negative resection while mitigating postoperative complications associated with splenic vessel resection in pediatric SPN cases.
A significant concern associated with Lap-WT involves postoperative complications, notably splenic infarction and the potential development of perigastric varices, particularly in pediatric cases. Postoperative splenic infarctions were not observed in our study. Initially, the primary collateral supply after splenic vessel resection, as described in the original report of the Warshaw procedure, was thought to be from the short gastric vessels. However, subsequent experimental and clinical investigations identified the left gastroepiploic artery and vein as the primary collateral vessels to the spleen after Warshaw procedure. Hence, it is crucial to dissect the pancreatic parenchyma meticulously from the bifurcation point of the distal splenic vessels encompassing the root of the left gastroepiploic artery at the splenic hilum during Lap-WT. Niec et al. were the first to report two pediatric SPN cases who underwent Lap-WT, with a median follow-up of 23 months after surgery, worldwide. Their surgical procedure emphasized the crucial importance of preserving the vasa brevia, specifically short gastric vessels, to maintain spleen perfusion. However, in their case series, both patients were reported to develop postoperative central splenic infarction, with postoperative CT scans showing a reduction in spleen volume of 65% and 74% compared with the preoperative volume, likely due to splenic atrophy resulting from focal splenic infarction. In Lap-WT, our tail-first approach prioritizes meticulous dissection of the pancreatic tail parenchyma from the splenic hilum to precisely isolate distal splenic vessels during the initial surgical stages. This technique facilitates the preservation of both roots of the left gastroepiploic artery and the short gastric artery, enabling a more precise stapler division of the distal splenic vessels. To the best of our knowledge, this is the first report of Lap-WT in pediatric patients where splenic perfusion was successfully maintained without any occurrence of splenic infarction, as confirmed by postoperative CT scans.
In the context of selecting the surgical approach for pediatric SPN located in the left-sided pancreas, laparoscopic DP with preservation of the splenic vessels (Lap-SVP) emerges as a feasible procedural alternative to Lap-WT for pediatric SPN.However, there remains controversy regarding which surgical approach is superior in terms of technical aspects and oncological outcomes for pediatric SPN. Given the potential for highly malignant types of SPN, referred to as high-grade SPN even in pediatric SPN, which exhibit distinct pathological features involving lymphatic and/or venous invasion, Lap-SVP might entail the risk of inadequate surgical margin clearance, particularly in cases of large SPN with involvement of the splenic vessels. Hence, our findings suggest that Lap-WT can be considered instead of Lap-SVP, even in pediatric cases of large SPN with tumor involvement of the splenic vessels. Conversely, Lap-WT is considered unsuitable for large SPN involving the vessels of the splenic hilum or those directly affecting the splenic parenchyma or cases with the presence of pancreatitis of the pancreatic tail with or without pseudocyst located at the hilum of the spleen. Further research is warranted to establish the optimal surgical strategy based on the clinicopathological characteristics for pediatric SPN.
In conclusion, our findings demonstrate that Lap-WT is a technically feasible and ontologically safe approach for pediatric patients with SPN, enabling margin-negative resection while minimizing complications associated with splenic vessel resections.




